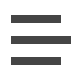On July 17th, the FDA announced approval of BeyfortusTM (nirsevimab) for prevention of respiratory syncytial virus (RSV) infections. Nirsevimab is a monoclonal antibody delivered intramuscularly as a single-dose that demonstrated 70-75% efficacy at reducing medically attended RSV lower respiratory tract infections for preterm and term infants in clinical trials1,2. Nirsevimab is classified as a drug by the FDA and is not a vaccine. Despite this designation, the Advisory Committee on Immunization Practices (ACIP) unanimously recommended the addition of nirsevimab to the Centers for Disease Control and Prevention’s (CDC’s) child immunization schedule and Vaccines for Children (VFC) program on August 3rd. The CDC recommends one dose of nirsevimab for all infants younger than 8 months, born during or entering their first RSV season. Additionally, it is recommended for children 8 to 19 months old who are at increased risk for severe RSV disease during their 2nd RSV season. Nirsevimab is expected to be available this fall. An initial summary of nirsevimab is provided below. As the 2023-2024 RSV season approaches, Partners For Kids will continue to provide nirsevimab updates in future newsletters.
- Griffin, M. P., et al. (2020). “Single-Dose Nirsevimab for Prevention of RSV in Preterm Infants.” New England Journal of Medicine 383(5): 415-425.
- Hammitt, L. L., et al. (2022). “Nirsevimab for Prevention of RSV in Healthy Late-Preterm and Term Infants.” New England Journal of Medicine 386(9): 837-846.